A Visual Guide to the Bohr Diagram for Nitrogen
Learn how to draw the Bohr diagram for nitrogen (N) with this easy-to-follow guide. Understand electron shells, valence electrons, and atomic structure.
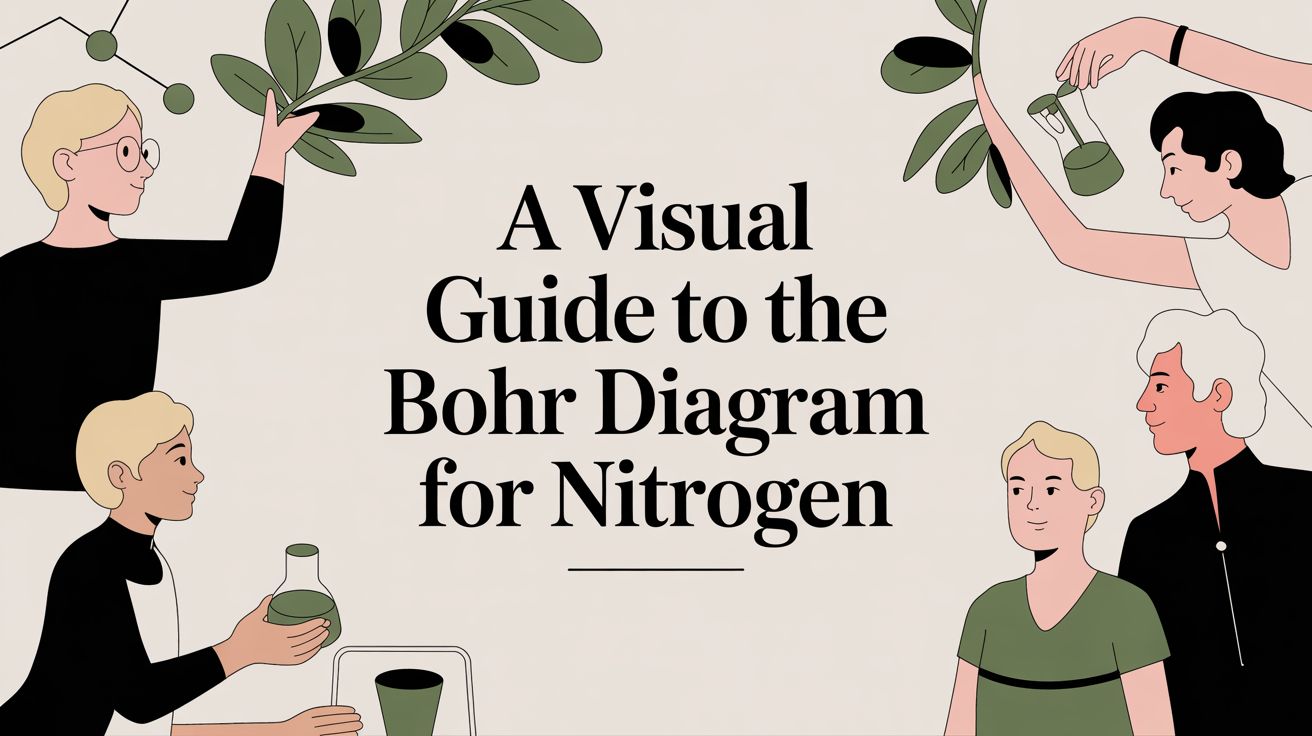
A Bohr diagram for nitrogen is a simple map of its atomic structure. It shows a nucleus with 7 protons at the center, circled by two energy shells. The inner shell has 2 electrons, while the outer shell holds the remaining 5 valence electrons. This little diagram is a surprisingly powerful tool for figuring out nitrogen's chemical behavior.
Mapping Out Nitrogen’s Atomic Structure
Before we put pencil to paper, we need to know what we're working with. The key to any element is its atomic number (Z), and for nitrogen, that magic number is 7.
This tells us everything we need to start: a neutral nitrogen atom has 7 protons in its nucleus and, to balance the charge, 7 electrons orbiting it.
The Bohr model gives us a straightforward way to organize these electrons. Developed by Niels Bohr back in 1913, it pictures electrons in fixed energy levels, or "shells," like planets orbiting the sun. It's a foundational concept that helps us visualize atomic structure, and you can dig deeper into its history over at Britannica.
Filling the Electron Shells
So, where do those 7 electrons go? There are a couple of basic rules for filling the shells, starting from the inside out.
- The first shell (closest to the nucleus) is tiny; it can only hold a maximum of 2 electrons.
- The second shell is bigger and can hold up to 8 electrons.
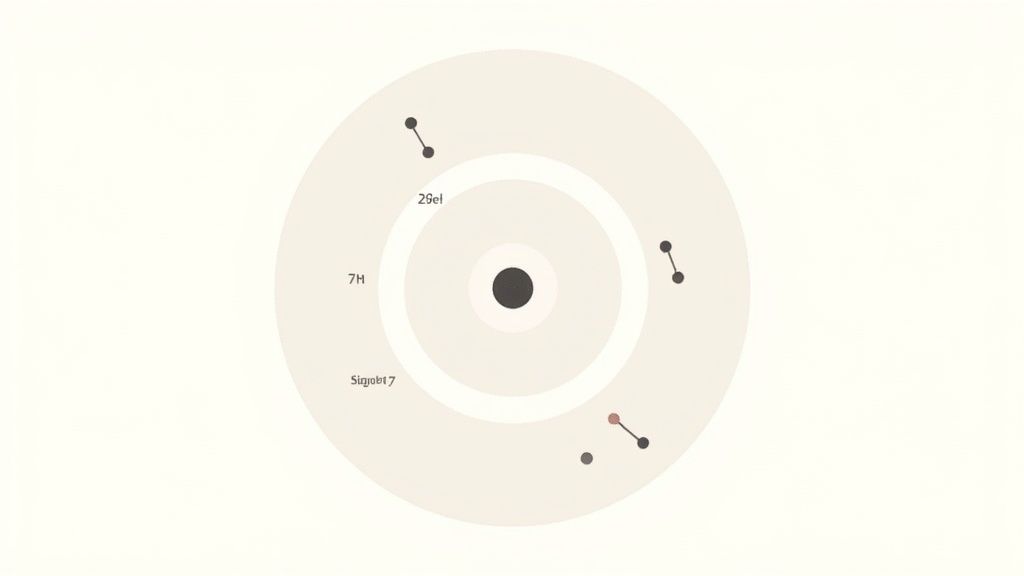
For nitrogen, we just need to place its 7 electrons according to these rules. The first 2 fill up that inner shell completely. That leaves us with 5 electrons, which all go into the second shell.
This gives us an electron configuration of (2, 5), which is the exact blueprint we need for our drawing.
To make things even clearer, here’s a quick summary of the essential data points for a neutral nitrogen atom.
Nitrogen Atomic Properties at a Glance
| Property | Value | Significance for the Diagram |
|---|---|---|
| Atomic Number (Z) | 7 | Tells us there are 7 protons and 7 electrons. |
| Total Electrons | 7 | These are the particles we need to place in shells. |
| Electron Shells | 2 | Nitrogen's electrons fit into two energy levels. |
| Electron Configuration | 2, 5 | 2 electrons in the first shell, 5 in the second. |
| Valence Electrons | 5 | The electrons in the outermost shell that drive bonding. |
This table neatly packages all the information you'll translate into your visual diagram.
Why This Matters: Those 5 electrons in the outer shell are called valence electrons. They're the most important ones because they're the ones that interact with other atoms. Understanding this is the first step to predicting how nitrogen forms bonds to create molecules essential for life, like ammonia (NH₃) and the amino acids in our DNA.
Figuring Out Nitrogen's Electron Layout
Alright, let's get our hands dirty and figure out where nitrogen's electrons actually go. Think of it as filling up seats in a tiny, exclusive theater. The best seats—the ones closest to the stage (the nucleus)—have to be filled first.
Nitrogen has a total of 7 electrons to work with. The first "row," or energy shell, only has two seats. So, we place the first 2 electrons there, filling it up completely. Easy enough.
That leaves us with 5 electrons still looking for a spot. They'll all go into the next shell out, which can hold up to eight. So there's plenty of room for them.
Nailing Down the Configuration
Just like that, we've found nitrogen's electron configuration: (2, 5). This simple notation is your blueprint. It tells you exactly how many electrons are in each shell, starting from the one closest to the center.
- First Shell: Has 2 electrons (it's full).
- Second Shell: Gets the remaining 5 electrons.
This isn't some special trick just for nitrogen. It's the basic rule for figuring out the electron layout for many other elements. Always fill the shells from the inside out. Get that down, and you'll nail the structure every time.
Your Drawing Blueprint: That (2, 5) configuration is the most important part. It literally tells you what to draw: a nucleus, a first circle with two dots, and a second circle with five dots.
Why That Outer Shell Is a Big Deal
So, why do we care so much about this (2, 5) layout? It all comes down to the electrons in the outermost shell. For nitrogen, those 5 valence electrons are what drive all its chemical reactions. They're the ones that get involved in bonding.
Having five electrons in its outer shell means nitrogen is just three shy of a full, stable set of eight (what chemists call a "stable octet"). This is what makes nitrogen so eager to react and form compounds. It's constantly looking to grab three more electrons to feel complete, which is why it's a key player in molecules like ammonia (NH₃) and even the DNA in our bodies.
Drawing the Bohr Diagram for Nitrogen
Now that we have the electron configuration mapped out, it's time for the fun part: translating that information into a visual model. Drawing a Bohr diagram is a simple, methodical process that brings the structure of a nitrogen atom to life.
Let's start from the inside out. Begin by drawing a small circle in the center of your workspace—this is the atom's nucleus. Inside this circle, we need to account for the protons and neutrons. For nitrogen's most common isotope (nitrogen-14), that means labeling "7p+" for its seven protons and "7n" for its seven neutrons.
Placing the Electrons on Their Shells
With the nucleus in place, it’s time to add the electron shells, which are just concentric circles around it. Draw the first circle, which represents the first energy level. We know from our (2, 5) configuration that this shell holds two electrons. I usually represent these as small dots placed on the circle.
Next, draw a second, larger circle around the first. This is nitrogen's outermost shell, and it's where things get interesting. This shell will hold the remaining five electrons.
A Quick Tip from Experience: When you place those five electrons, don't just clump them all together. It's much clearer if you space them out. A common convention is to place one electron at the 12, 3, 6, and 9 o'clock positions first. Then, you can add the fifth electron next to any of the others. This creates a single "pair" and three unpaired electrons, which is a great visual cue for understanding bonding.
Following this simple spacing technique makes your Bohr diagram for nitrogen far easier to interpret at a glance. It clearly shows the five valence electrons available for chemical reactions.
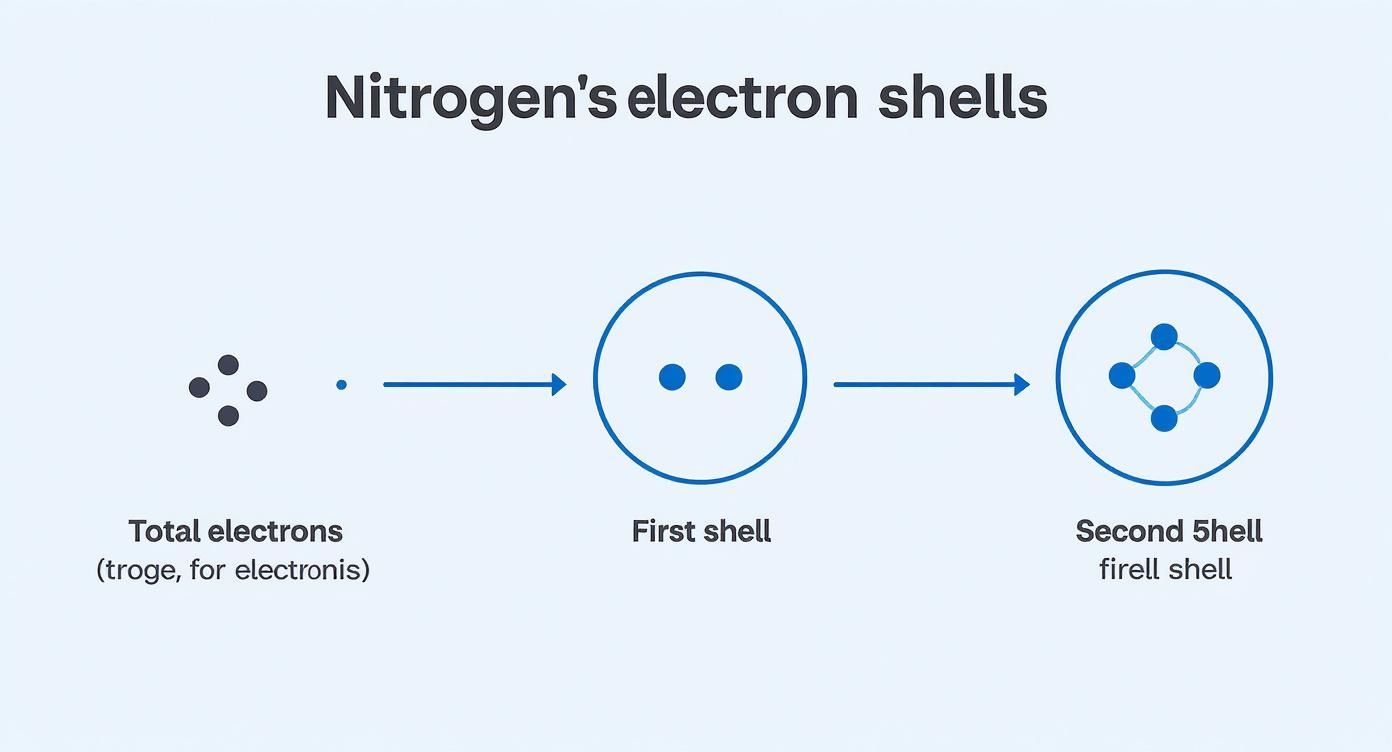
The infographic above provides a great summary of this process, showing how nitrogen’s seven electrons first fill the inner shell to capacity before populating the second one.
What Your Completed Diagram Tells You
Take a step back and look at your completed drawing. It’s more than just circles and dots; it’s a functional map of nitrogen's chemical behavior. The key to understanding that behavior lies in those five electrons you drew on the outermost ring.
These are the valence electrons, and they are the drivers of chemical bonding. The second shell is most stable when it holds a full set of eight electrons. With five already there, nitrogen is just three electrons shy of that stable state. This powerful drive to gain three more electrons is precisely what makes nitrogen so reactive and a cornerstone element for life itself. Your diagram is a perfect snapshot of this fundamental chemical principle.
So What Does This Diagram Actually Tell Us?
A finished Bohr diagram for nitrogen isn't just a classroom exercise—it’s a visual key to understanding the atom's chemical personality. The real story is told by those five electrons in the outermost shell.
These are the valence electrons, and they’re the ones calling all the shots. They dictate almost everything about how nitrogen will behave and interact with other elements.
Think of it this way: atoms are always chasing stability. The ultimate goal for most is a full outer shell of eight electrons. This core concept, known as the octet rule, is one of the most fundamental principles in chemistry.
Why Nitrogen Is So Eager to Bond
When you look at the diagram, nitrogen has five valence electrons. It's so close, yet so far—just three electrons short of that stable octet. This "incompleteness" is what makes nitrogen reactive. It’s an atom on a mission, actively looking to gain or share three more electrons to finally feel complete.
This powerful drive is the "why" behind some of nitrogen's most common chemical behaviors.
- Forming N₂ Gas: It’s the reason two nitrogen atoms will grab onto each other and form an incredibly strong triple bond. They share three pairs of electrons, satisfying each other's octets and creating the super-stable N₂ gas that makes up 78% of the air we breathe.
- Making Ammonia (NH₃): It also explains why a nitrogen atom will happily bond with three hydrogen atoms. Nitrogen shares one of its electrons with each hydrogen, and each hydrogen shares its one electron back. It's a perfect arrangement where everyone gets what they need to be stable.
In short, the simple visual of five dots in that outer ring explains why nitrogen is a cornerstone of life itself, forming the backbone of crucial molecules like amino acids and DNA.
From Drawing to Predicting
Once you grasp this connection, you can go from just drawing a diagram to actually predicting how an element will act. The moment you see those five valence electrons on a nitrogen atom, you can confidently guess that it will want to form three bonds to stabilize itself.
This predictive power is what makes the Bohr model such a valuable tool, especially when you're starting out. It takes the abstract idea of electron shells and makes it tangible, linking it directly to real-world chemical properties and reactions. Your drawing isn't just an assignment—it’s a map to understanding chemistry on a much deeper level.
Watching Out for Common Pitfalls
When you're first getting the hang of Bohr diagrams, a few common trip-ups can happen. It's all part of the process. Getting the Bohr diagram for nitrogen right is mostly about dodging these specific, easy-to-make mistakes.
One of the biggest hurdles I see students face is putting too many electrons in that first, innermost shell. It's a hard and fast rule: that first energy level can only hold a maximum of two electrons. That's it. Once those two spots are filled, you have to move to the next shell.
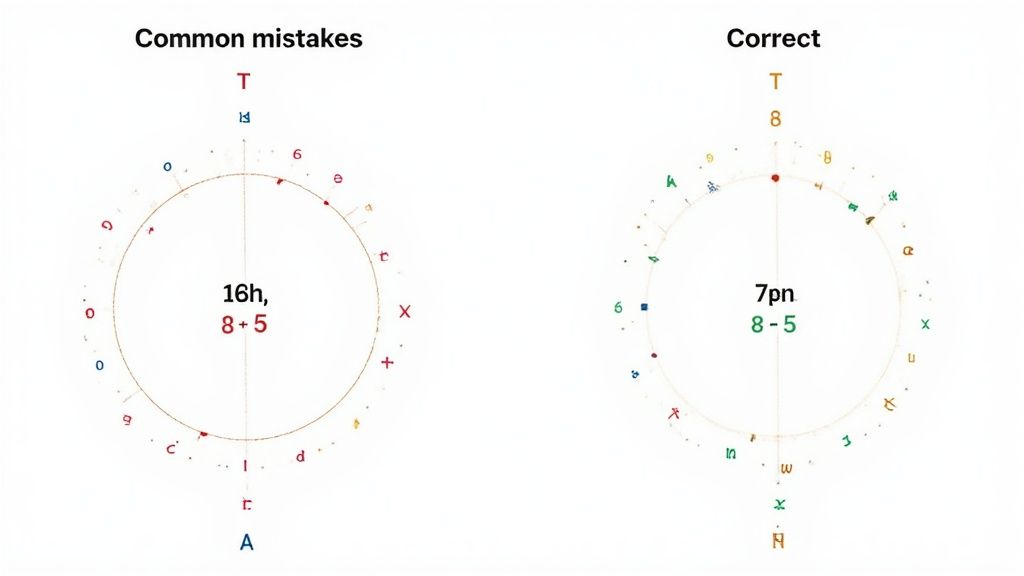
Simple Errors in Counting and Labeling
Another classic mistake is a simple miscount of the total electrons. Remember, for a neutral nitrogen atom, the number of electrons must be identical to the number of protons, which is 7. Always do a quick final count—did you place exactly seven dots on your diagram?
And don't forget the nucleus! It’s the anchor of the whole diagram. Forgetting to label it with "7p+" is a common oversight. That little label is crucial because it signifies the positive charge that's holding those negatively charged electrons in orbit.
Expert Tip: When you get to the five valence electrons, try not to just clump them all together. A much better habit is to spread them out evenly around the outer shell first, almost like placing numbers on a clock, before you pair any of them up. This not only makes your diagram much cleaner but also gives you a better visual cue for how nitrogen actually forms bonds.
Common Questions About Nitrogen's Bohr Diagram
Even after drawing a few of these, some questions always seem to pop up. Let's clear up a few of the most common sticking points I see with students when they're first tackling the Bohr diagram for nitrogen.
Why Does the First Shell Only Hold Two Electrons?
This is a great question. Think of it like this: the first energy shell (often labeled n=1) is the smallest and closest to the nucleus, so it has the least amount of space and the lowest energy.
Quantum mechanics gets into the weeds, but the simple version is that this first shell only has one type of "room" for electrons, called an 's' orbital. Any single orbital can only hold a maximum of two electrons. That's the hard limit, which is why it fills up right away before any electrons can start populating the second, larger shell.
How Would a Nitrogen Ion Look Different?
A standard nitrogen atom is neutral, meaning it has 7 protons and 7 electrons. But nitrogen often forms the N³⁻ ion by gaining 3 electrons. It does this to achieve a super-stable, full outer shell.
If you were to draw its Bohr diagram, you'd see:
- The nucleus remains unchanged with 7p+.
- The first shell is still full with 2 electrons.
- The second shell now has a full set of 8 electrons (the original 5 plus the 3 it gained).
- Crucially, you'd bracket the whole diagram and add a [3-] charge to show it's no longer a neutral atom.
The Bohr model is a fantastic educational tool for visualizing electron shells and valence electrons. While it's a simplification—the modern quantum model describes electrons in 3D probability clouds called orbitals—it remains an essential concept for introductory chemistry because of its clarity and predictive power.
Is the Bohr Diagram the Most Accurate Model?
Honestly, no. But it's incredibly useful for what it does. The Bohr model is a simplified map that helps us grasp foundational concepts like energy levels and how valence electrons work. It's a stepping stone.
For higher-level chemistry, you'll move on to the quantum mechanical model, which gives a much more precise (and complex!) picture of electrons existing in 3D orbitals rather than neat, flat orbits. For anyone just starting out, though, mastering the Bohr diagram for nitrogen is the perfect place to build your foundation.
Struggling with complex chemistry concepts or need quick help with your homework? Feen AI can break down tough problems step-by-step. Upload a photo of your assignment and get clear explanations for Chemistry, Physics, Math, and more.
Relevant articles
Discover an easy mass to moles conversion method with clear steps, practical examples, and pitfalls to avoid—boost your chemistry confidence today.
