Spotting Reactions: 5 Signs of a Chemical Change Explained
Unlock the secrets of chemistry with our guide to the 5 signs of a chemical change. Learn to identify reactions through color, heat, gas, and more!

Ever wondered how a campfire transforms wood into ash, or how baking soda and vinegar create a fizzing volcano? These transformations are not magic; they are chemistry in action, representing fundamental shifts at a molecular level. The world around us is in a constant state of flux, but not all changes are created equal. Some are merely physical, like ice melting into water, where the substance's identity remains the same. Others, however, are profound and often irreversible.
These are chemical changes, where substances rearrange their atomic bonds to form entirely new materials with completely different properties. But how can you tell when such a reaction is actually happening, especially when you can't see the atoms themselves? Fortunately, the universe provides clear, observable clues. This guide is designed to make you a scientific detective, equipped to spot these transformations everywhere.
We will explore the definitive 5 signs of a chemical change, moving beyond simple definitions to provide you with practical examples, from the kitchen to the laboratory. Understanding these signs is the first step toward decoding the chemical reactions that shape our world, offering a powerful lens through which to view everyday phenomena. You will learn to identify these key indicators confidently and distinguish them from simple physical alterations.
1. Color Change: The Visual Clue
Of the five signs of a chemical change, a sudden and unexpected color change is often the most dramatic and easiest to spot. This visual clue indicates that the fundamental structure of the substances involved has been altered. The original molecules have broken their bonds and rearranged their atoms to form entirely new substances with different properties.
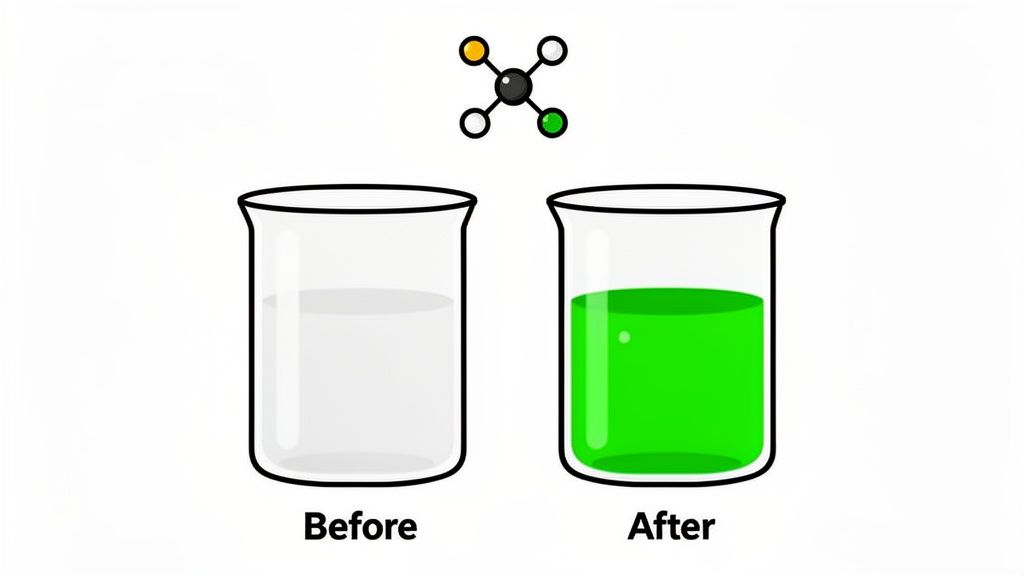
This phenomenon occurs because the new molecules absorb and reflect light differently than the original reactants. It's a fundamental shift in chemical identity, not just a physical mixing like adding red food coloring to water.
Why Does Color Change Signal a Chemical Reaction?
The color of a substance is determined by its molecular structure and how it interacts with the visible light spectrum. When a chemical reaction happens, the electron configurations of the atoms change as new chemical bonds are formed. These new configurations cause the resulting product to absorb different wavelengths of light, reflecting back a new color to our eyes. For example, when shiny, metallic iron is exposed to oxygen and water, it undergoes oxidation to form a new compound, iron(III) oxide. This new substance has a completely different structure that reflects reddish-brown light, which we recognize as rust.
Examples in Everyday Life
You don't need a lab coat to see this sign of a chemical change; it happens all around you.
- Fruit Browning: An apple slice turns brown when exposed to air due to an enzyme-driven reaction called oxidation.
- Patina on Copper: The Statue of Liberty wasn't always green. It's made of copper, which has oxidized over time to form a greenish-blue patina.
- Silver Tarnishing: A shiny silver spoon slowly develops a black coating (silver sulfide) when it reacts with sulfur compounds in the air.
- Cooking an Egg: The clear egg white turns opaque and white as the proteins denature and rearrange due to heat.
A fascinating biological example is the changing color of leaves in autumn. As trees prepare for winter, they stop producing chlorophyll, the molecule responsible for their green color. This process, tied to the complex chemical dance of photosynthesis, allows other pigments like carotenoids (yellows and oranges) to become visible. You can discover more about the intricate chemistry of photosynthesis and how it drives life on our planet.
Key Distinction: Remember, mixing two colors of paint is a physical change. The paint pigments are just interspersed, not chemically altered. A true chemical color change involves the creation of a new substance.
Classroom Demonstration: The "Golden Rain" Reaction
A classic and visually stunning experiment is the reaction between potassium iodide and lead(II) nitrate solutions. When these two clear, colorless liquids are mixed, they instantly form a vibrant yellow solid precipitate (lead(II) iodide). This immediate and dramatic color change is a clear indicator that a new substance has been created, demonstrating one of the key signs of a chemical change in action.
2. Temperature Change: Feeling the Energy Flow
Beyond visual cues, a noticeable change in temperature is a powerful and direct sign of a chemical change. This thermal shift reveals that energy is being transferred as chemical bonds are broken and reformed. The process isn't just about rearranging atoms; it’s also about the fundamental flow of energy, which is either released into the surroundings or absorbed from them.
This energy transfer occurs because different chemical bonds store different amounts of energy. When the bonds in the reactants are broken and new, more stable bonds are formed in the products, excess energy is released, usually as heat. Conversely, if the new bonds are less stable, energy must be absorbed from the environment to drive the reaction forward.
Why Does Temperature Change Signal a Chemical Reaction?
At the heart of any chemical reaction is the rearrangement of chemical bonds, and this process always involves energy. Reactions that release energy are called exothermic reactions, and they cause the temperature of the surroundings to increase. You can feel this as heat. On the other hand, reactions that absorb energy from their surroundings are called endothermic reactions, and they cause the temperature to drop, making things feel cold. This measurable change in thermal energy is a clear indicator that the original substances have been transformed into new ones with different energy states. It's a fundamental principle of chemical thermodynamics in action.
Examples in Everyday Life
You've undoubtedly encountered this sign of a chemical change many times without realizing the chemistry behind it.
- Burning Wood: A campfire is a classic example of an exothermic reaction. The combustion of wood releases a large amount of energy as heat and light.
- Hand Warmers: Disposable hand warmers use the slow oxidation of iron to generate heat, a perfect example of a controlled exothermic reaction.
- Cold Packs: Instant cold packs utilize an endothermic reaction. When you break the inner pouch, ammonium nitrate dissolves in water, absorbing heat from the surroundings and making the pack feel icy cold.
- Cooking Food: Frying an egg or baking a cake involves chemical reactions driven by heat. The change in texture and taste is a result of new chemical compounds being formed.
A critical biological example is cellular respiration. In our bodies, glucose and oxygen react to produce carbon dioxide, water, and energy. This series of exothermic reactions is what maintains our body temperature and provides the energy we need to live.
Key Distinction: Boiling water is a physical change, not a chemical one. Although the temperature increases, the water molecules (H₂O) are not changing their chemical identity; they are simply transitioning from a liquid state to a gaseous state (steam).
Classroom Demonstration: The "Volcano" Reaction
A simple and safe demonstration is the reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid). When you mix these two common household items, they immediately begin to fizz and bubble, producing carbon dioxide gas. If you place a thermometer in the mixture, you will observe a noticeable drop in temperature. This is a classic endothermic reaction, providing clear, measurable evidence of a chemical change taking place.
3. Gas Evolution (Gas Production): The Bubbling Evidence
Among the five signs of a chemical change, the production of a gas is a dynamic and often unmistakable indicator. Referred to as gas evolution, this sign involves the formation of a gaseous substance from solid or liquid reactants. The appearance of bubbles, fizzing, or an inflating balloon signifies that the original substances have rearranged their atoms to form a new compound in a gaseous state.
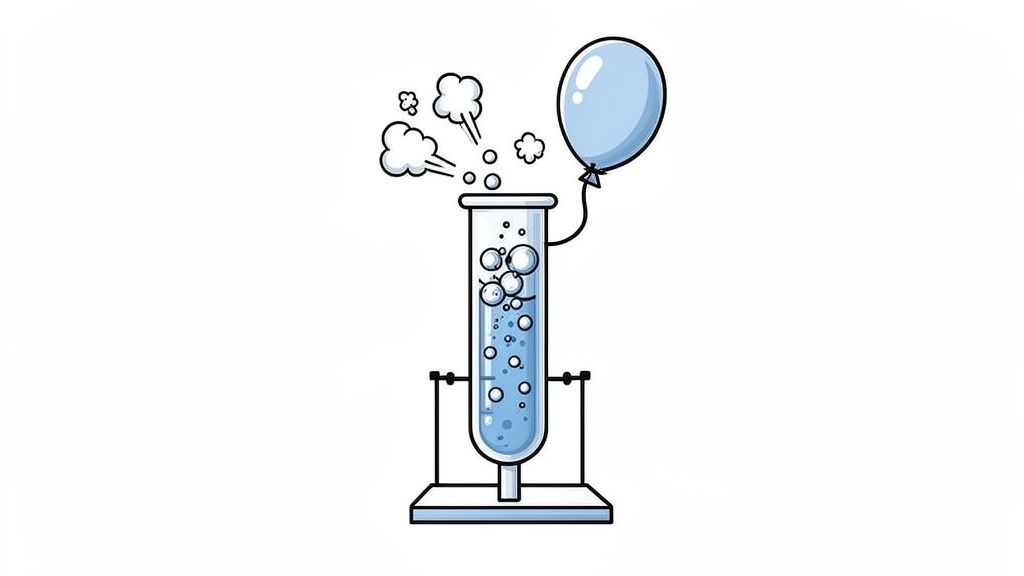
This process is a clear signal of chemical transformation because it involves a change in the state of matter resulting from the creation of a new substance. Unlike simply boiling water (a physical change), the gas produced during a chemical reaction is a completely different compound than the reactants that started the process.
Why Does Gas Production Signal a Chemical Reaction?
Gas evolution signals a chemical change because the bonds within the reactant molecules are broken and new bonds are formed, creating a product with different physical properties, specifically a substance that exists as a gas at the reaction temperature. For a new substance to form as a gas, a significant rearrangement of atoms must occur. For instance, when an acid reacts with a carbonate, the carbonate compound breaks down, and its carbon and oxygen atoms rearrange to form carbon dioxide (CO₂) gas, a molecule with completely different characteristics from the original solid carbonate. The release of this gas is a definitive sign that a new chemical identity has been forged.
Examples in Everyday Life
Gas production is a common chemical change that you can observe in many familiar settings, from the kitchen to the natural world.
- Baking: The leavening agents in baking, like baking soda (sodium bicarbonate), react with acidic ingredients to produce carbon dioxide gas. These bubbles get trapped in the dough, causing it to rise.
- Effervescent Tablets: Dropping an antacid or vitamin tablet into water produces vigorous fizzing. This is a chemical reaction producing CO₂ gas.
- Fermentation: The process yeast uses to convert sugars into alcohol also produces carbon dioxide. This is responsible for the bubbles in beer and champagne and helps bread rise.
- Digestion: The breakdown of food in your stomach involves acids that can produce gas as a byproduct.
A powerful natural example is decomposition. When organic matter breaks down, microorganisms carry out chemical reactions that release gases like methane and carbon dioxide. Understanding the stoichiometry of these reactions is crucial, and you can master the skill of balancing chemical equations to better understand these processes.
Key Distinction: Boiling water to create steam is a physical change. The H₂O molecules are still H₂O, they are just moving faster and are farther apart. Gas evolution in a chemical change creates a new substance, like when vinegar and baking soda create CO₂ gas.
Classroom Demonstration: The "Volcano" Reaction
A simple, safe, and iconic demonstration of gas production is the reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid). When these two common household items are mixed, they react immediately to produce carbon dioxide gas, water, and sodium acetate. The vigorous bubbling is the CO₂ gas escaping. For a dramatic effect, this reaction can be performed inside a model volcano with a bit of soap and food coloring to create a foamy "lava" flow, clearly showing gas production as one of the key signs of a chemical change.
4. Formation of a Precipitate: A Solid Surprise
Observing a solid suddenly appear in a clear solution is a compelling sign that a chemical change has occurred. This solid, known as a precipitate, forms when two soluble reactants in a solution combine to create a new substance that is insoluble in the liquid. The appearance of this new solid phase, whether as a cloudy suspension, distinct particles, or a settled layer, is a direct result of atoms rearranging to form new compounds with different physical properties.
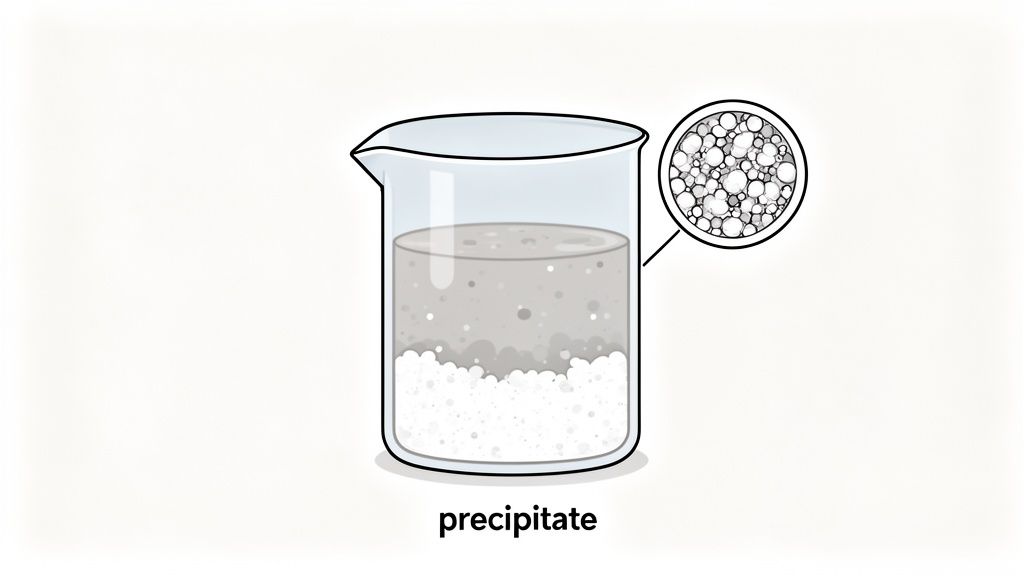
This process demonstrates that the original substances have undergone a fundamental transformation. The ions that were once freely dissolved have now bonded together so strongly that they separate from the solution, providing visible, tangible proof of a new substance being created.
Why Does a Precipitate Signal a Chemical Reaction?
Precipitation reactions are driven by the principles of solubility and ionic bonding. In a solution, the ions of soluble compounds are dissociated and move freely. When two such solutions are mixed, different combinations of positive and negative ions become possible. If a new combination of ions forms a compound that is insoluble in the solvent (usually water), these ions will bond together to form a solid lattice structure, which then precipitates out. This is a clear indicator of chemical change because the new solid has a completely different chemical identity and properties, like solubility, from the original reactants.
Examples in Everyday Life
While common in the chemistry lab, precipitation reactions also happen in more familiar settings.
- Milk Curdling: Adding an acid like lemon juice or vinegar to milk causes the casein proteins to denature and clump together, forming solid curds that separate from the liquid whey.
- Hard Water Deposits: The white, chalky scale that builds up in kettles and pipes in areas with hard water is a precipitate of calcium carbonate and magnesium carbonate, formed from dissolved minerals in the water.
- Kidney Stones: In a biological context, kidney stones are solid precipitates formed from minerals like calcium oxalate that crystallize out of urine.
- Making Cheese: The fundamental process of cheesemaking involves the controlled precipitation of milk proteins to form curds.
A great example from industrial chemistry is the production of certain pigments and compounds. For instance, mixing copper sulfate and sodium hydroxide solutions produces a distinct blue precipitate. You can explore the chemical makeup and uses of this compound to better understand the copper hydroxide formula and its properties.
Key Distinction: A solid settling out of muddy water is not a chemical change. This is a physical process called sedimentation, where insoluble particles that were merely suspended in the water fall due to gravity. No new substance is formed.
Classroom Demonstration: The "Silver Mirror" Precursor
A simple yet effective demonstration involves mixing solutions of silver nitrate (AgNO₃) and sodium chloride (NaCl), also known as table salt. Both are clear, colorless liquids. Upon mixing, they immediately form a thick, white precipitate of silver chloride (AgCl), an insoluble solid. This instantaneous formation of a solid from two clear liquids is an undeniable sign of a chemical change, showcasing how new ionic bonds have created an entirely new substance.
5. Production of Light and Sound: The Energetic Signal
Perhaps the most dramatic and exciting of the five signs of a chemical change are the production of light and sound. These energetic signals indicate that a reaction is releasing a significant amount of energy, often very quickly. When you see a sudden flash of light or hear a distinct pop or fizz, it's a powerful clue that chemical bonds are being broken and new, more stable bonds are being formed, releasing the excess energy into the environment.
This phenomenon demonstrates that chemical changes aren't just about rearranging atoms to make new substances; they are also fundamentally about transforming energy. The release of energy as light or sound is a direct consequence of the molecular-level restructuring that defines a chemical reaction.
Why Do Light and Sound Signal a Chemical Reaction?
The production of light and sound is a hallmark of exothermic reactions, which are reactions that release energy. Light is produced when the reaction gives off energy in the form of photons. This can happen through incandescence (light from heat, like a flame) or chemiluminescence (light produced by the chemical reaction itself without significant heat).
Sound production, such as a pop or fizz, is typically caused by the rapid expansion of gas. If a reaction produces a gas very quickly, the gas molecules push forcefully against the surrounding air, creating a pressure wave that our ears interpret as sound. An explosive reaction is simply an extremely fast version of this process, releasing a massive amount of gas and energy almost instantly.
Examples in Everyday Life
You've likely witnessed these energetic signs of a chemical change without even realizing the complex chemistry at play.
- Fireworks: The vibrant colors and loud bangs of fireworks are a perfect example, resulting from the rapid combustion of metal salts and oxidizing agents.
- Striking a Match: The scratch ignites a reaction on the match head, producing a flame (light and heat) and a distinct crackling sound.
- Combustion: Any burning process, from a campfire to the engine in a car, releases energy as light (the flame) and sound (the crackle or roar).
- Glow Sticks: These fun items use chemiluminescence. Bending the stick mixes two chemicals, initiating a reaction that releases energy directly as light, without producing heat.
Key Distinction: Remember, flicking a light switch produces light, but it is a physical process. Electricity is flowing through a filament, causing it to glow from heat. No new substance is created. A chemical change, like in a glow stick, produces light because a new substance is being formed.
Classroom Demonstration: The "Whoosh Bottle"
A classic demonstration involves swirling a small amount of flammable alcohol (like isopropyl alcohol) inside a large plastic water jug to create a vapor-air mixture. When a lit match is brought to the opening, the vapor ignites with a dramatic "whoosh" sound and a large, visible flame. This rapid combustion reaction powerfully demonstrates how the formation of new products (carbon dioxide and water) can release a tremendous amount of energy as both sound and light, clearly signaling a chemical change. (Note: This demonstration should only be performed by a qualified educator with proper safety precautions.)
Comparison of 5 Signs of Chemical Change
| Indicator | Implementation Complexity | Resource Requirements | Expected Observable Outcome | Ideal Use Cases | Key Advantages | Key Limitations / Safety |
|---|---|---|---|---|---|---|
| Color Change | Low — simple observation | Minimal — good lighting, optional camera | Visible shift in color (qualitative) | Introductory labs, quick demos, visual identification | Immediate, engaging, instrument-free | Subjective, affected by lighting; not quantitative; some changes subtle |
| Temperature Change | Low–Medium — requires measurement | Thermometer/thermal probe, controlled setup | Measurable temperature rise or drop (quantitative) | Thermochemistry, kinetics, distinguishing exo-/endothermic reactions | Quantifiable data; links to energy changes | Requires equipment; heat loss/environmental effects; slow changes hard to detect |
| Gas Evolution (Gas Production) | Low–Medium — observe or collect gas | Glassware, tubing/collection apparatus, ventilation | Bubbling, effervescence, odor, balloon inflation; gas identifiable | Stoichiometry, mechanism studies, fermentation/baking demos | Highly visible, testable gases, shows irreversibility | Possible toxic/flammable gases; collection/measurement can be tricky; safety precautions needed |
| Formation of a Precipitate | Low — mix solutions and observe | Reagents, basic glassware, optional filtration setup | Cloudiness or solid settling out of solution (solid formed) | Analytical tests, solubility teaching, ion identification | Clear, isolatable evidence; useful for qualitative analysis | Requires solubility knowledge; slow or dissolving precipitates can confuse results |
| Production of Light and Sound | Medium–High — controlled reactions | Specific reactants, safety equipment, controlled environment | Bright light, flashes or audible pops/crackles (energetic) | Demonstrations of energy release, advanced labs, pyrotechnics study | Dramatic, memorable multi-sensory evidence of high energy release | High hazard potential; often impractical in classrooms; requires strict safety controls |
From Observation to Understanding: Your Next Steps in Chemistry
Navigating the world of chemistry begins with a single, crucial skill: observation. Throughout this guide, we've unpacked the 5 signs of a chemical change, transforming them from abstract textbook terms into tangible, real-world indicators. By recognizing a sudden color change in a rusting nail, feeling the heat from an exothermic reaction, or seeing the fizz of gas bubbles when baking soda meets vinegar, you are directly witnessing the rearrangement of atoms. These are not just isolated phenomena; they are the fundamental language of chemistry.
Mastering these signs moves you from a passive observer to an active participant in scientific inquiry. You now have a framework for asking why. Why did the solution turn cloudy? Why did the test tube get cold? The formation of a precipitate, a change in temperature, the production of light, the evolution of gas, and a shift in color are your primary clues. They are the evidence that old chemical bonds have been broken and new ones have been formed, creating entirely new substances with different properties.
Putting Your Knowledge into Practice
The true value of this knowledge lies in its application. Your journey into chemistry doesn't end with reading this article; it begins the next time you step into a kitchen, a lab, or even just look at the world around you with a more curious eye. The key is to consciously connect what you see with the underlying principles.
- In the Kitchen: When you cook an egg, observe the change in the white's color and texture. That’s a chemical change. When you bake a cake, notice the bubbles that help it rise; that’s gas evolution.
- In the Lab: Don't just follow the steps of an experiment. Actively look for the signs. Document whether a precipitate formed, if the beaker felt warm or cool, or if you noticed a faint fizzing sound.
- In Everyday Life: See a car's rusty fender? That's a slow chemical reaction (oxidation) with a clear color change. A lit match? That's the production of light and heat.
By actively identifying these 5 signs of a chemical change, you build a stronger, more intuitive understanding of how matter interacts. This skill is foundational, serving as the bedrock for more complex topics like stoichiometry, thermodynamics, and organic chemistry. To continue your journey of discovery and apply what you've learned, you can explore engaging practical exercises such as these 10 Fun Science Activities for Kids, which offer hands-on ways to see these principles in action.
Embrace your curiosity. The universe is a vast and dynamic laboratory, constantly undergoing chemical transformations. By learning to read its signs, you unlock a deeper appreciation for the intricate and beautiful processes that shape our world.
Struggling to interpret your lab results or need help understanding a complex reaction for your homework? Take a photo of your experiment or problem, and let Feen AI provide instant, step-by-step explanations. Get the clarity you need to master chemistry by visiting Feen AI today.
Relevant articles
Learn how to balance chemical equations with clear, step-by-step methods and practical examples to boost your stoichiometry skills.
