Empirical Formula for Ascorbic Acid Explained
Discover the empirical formula for ascorbic acid (C₃H₄O₃) with this clear, step-by-step guide. We break down the chemistry behind Vitamin C's simplest ratio.

The empirical formula for ascorbic acid—what you probably know as Vitamin C—is C₃H₄O₃. Think of this as the simplest chemical blueprint for this essential nutrient. It reveals the most basic whole-number ratio of carbon, hydrogen, and oxygen atoms inside the molecule.
Cracking the Code of Ascorbic Acid
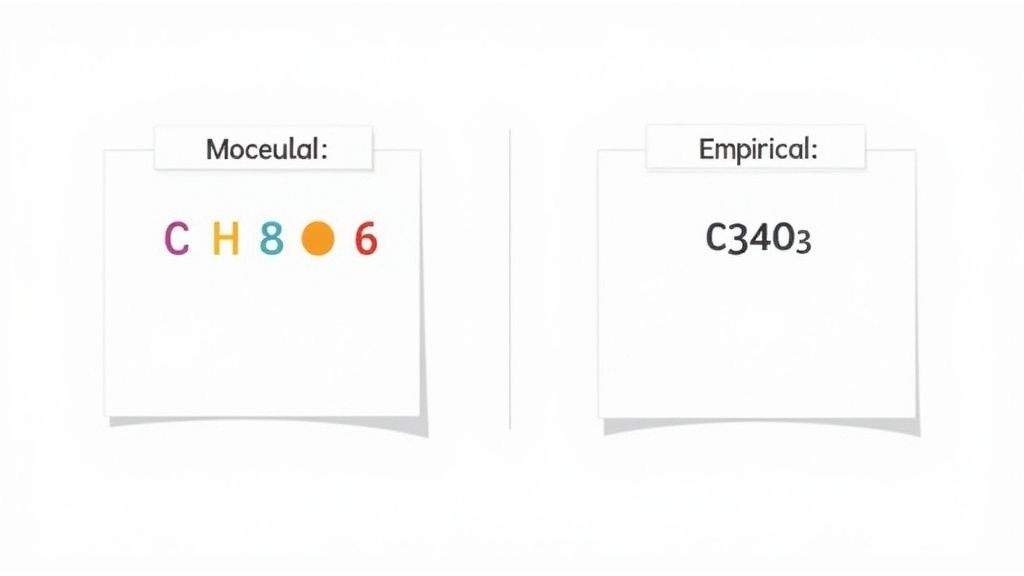
To really get what this formula means, we need to look at the two main ways chemists describe a compound's atomic makeup: the molecular formula and the empirical formula. Each one gives us a different piece of the puzzle.
The molecular formula for ascorbic acid is C₆H₈O₆. This is the exact headcount. It tells you precisely how many atoms of each element are packed into a single molecule: 6 carbon atoms, 8 hydrogen atoms, and 6 oxygen atoms. No more, no less. It's the true, complete count.
The empirical formula, C₃H₄O₃, on the other hand, is all about simplicity. It’s like a reduced fraction. It shows the simplest possible whole-number ratio of those atoms to each other.
The Baking Analogy
Let's imagine you have a cake recipe that calls for 6 cups of flour, 8 eggs, and 6 cups of sugar. That's your molecular formula—the exact ingredients needed for one cake.
The empirical formula just gives you the core ratio. If you divide all those numbers by their greatest common divisor (which is 2), you get 3 parts flour, 4 parts eggs, and 3 parts sugar. This simplified ratio, C₃H₄O₃, is the fundamental building block of the recipe.
Here's a quick way to keep them straight: The Molecular formula shows the Massive, true number of atoms. The Empirical formula shows the Essential, simplest ratio.
To quickly see the difference side-by-side, this table breaks down the two formulas for Vitamin C.
Ascorbic Acid At a Glance: Molecular vs. Empirical Formula
| Attribute | Molecular Formula (C₆H₈O₆) | Empirical Formula (C₃H₄O₃) |
|---|---|---|
| Meaning | The actual number of atoms in one molecule. | The simplest whole-number ratio of atoms. |
| Atom Count | 6 Carbon, 8 Hydrogen, 6 Oxygen | Ratio of 3 Carbon: 4 Hydrogen: 3 Oxygen |
| Primary Use | Identifies the specific compound and its mass. | Shows the relative proportions of elements. |
| Analogy | The full, precise recipe for one cake. | The basic ratio of ingredients (e.g., 3 parts flour to 4 parts eggs). |
This table highlights how the molecular formula gives the full picture, while the empirical formula provides the foundational ratio. Grasping this difference is the key to understanding how chemists analyze and identify substances.
Understanding the Concept of an Empirical Formula
Before we jump into finding the empirical formula for ascorbic acid, let's get a solid grip on what an empirical formula actually is. Think of it as a chemical compound's most simplified recipe. It shows the simplest whole-number ratio of atoms in the compound, not the total number of atoms in a single molecule.
Imagine you're building something with LEGOs. You use 10 red bricks and 20 blue bricks. The "molecular formula" would be the exact count: R₁₀B₂₀. The empirical formula, on the other hand, just boils that down to the basic ratio. For every one red brick, you have two blue ones. So, the empirical formula is simply RB₂. That’s it—it’s all about the simplest ratio.
This idea is fundamental in chemistry. While the molecular formula gives you the complete blueprint of a molecule, the empirical formula provides the essential, stripped-down ratio of its parts.
What an Empirical Formula Reveals
So, what does this simplified formula actually tell a chemist who's analyzing a substance? It gives two crucial pieces of information right away:
- Which elements are present: It identifies all the ingredients in the compound, like carbon, hydrogen, and oxygen.
- The simplest ratio: It shows how those atoms relate to each other proportionally in their most reduced form.
This is where it differs from the molecular formula, which gives you the actual number of atoms. Understanding how atoms are structured is key to predicting their behavior, much like how a Bohr diagram for nitrogen helps us see its electron shells. The empirical formula is often the very first clue we get on the path to figuring out the full molecular picture.
Key Takeaway: An empirical formula is like a recipe's base ratio (e.g., 2 parts flour to 1 part sugar). The molecular formula is the full recipe for a specific cake size (e.g., 4 cups of flour and 2 cups of sugar).
Ultimately, the empirical formula is like a chemical fingerprint. When scientists analyze a sample, this is often the first thing they can figure out from experimental data. For any chemistry student, getting this concept down is a huge step toward solving a whole range of problems.
The Difference Between Empirical and Molecular Formulas
https://www.youtube.com/embed/242aoPZuE68
To really get a handle on the empirical formula for ascorbic acid, we first need to draw a clear line between it and its molecular formula. They're two different ways of looking at the same molecule, kind of like the difference between a high-resolution photo and a simple sketch—both show you the subject, but with different levels of detail.
The molecular formula, C₆H₈O₆, is the whole story. It gives you the exact, non-negotiable count of every atom in a single molecule of ascorbic acid. Think of it as a precise recipe: you have 6 carbon atoms, 8 hydrogen atoms, and 6 oxygen atoms all working together. This formula is what you need to calculate the molecule's exact mass and understand its full structure.
On the other hand, the empirical formula, C₃H₄O₃, is the simplified version. It boils the molecule down to the lowest whole-number ratio of its atoms. It doesn't give you the total count, just the fundamental proportion.
From Real-World Data to Chemical Formulas
You might be wondering why we even have two different formulas. In the lab, chemists often figure out the empirical formula first. By running experiments to determine a compound's percent composition by mass, they can work backward to find the simplest ratio of elements. It's only after they determine the molecule's total molar mass that they can scale up that ratio to find the true molecular formula.
Here's a quick tip to keep them straight: The Molecular formula tells you the Massive, true count of atoms. The Empirical formula gives you the Essential, simplest ratio.
Let’s try one more analogy to lock this in. Imagine a small company has 12 engineers and 18 designers.
- The "Molecular" Count: The exact roster is E₁₂D₁₈. This tells you precisely how many people are in each role.
- The "Empirical" Ratio: To get the simplest ratio, we find the greatest common divisor, which is 6. Dividing both by 6 gives us E₂D₃. This tells us that for every 2 engineers, there are 3 designers.
That simplified ratio is the empirical view. It gives you the core relationship between the parts without telling you the total size of the team. In chemistry, both formulas are incredibly useful. The empirical formula is often the first clue you get from an experiment, while the molecular formula provides the complete picture needed to truly understand a compound like ascorbic acid.
How to Find the Empirical Formula for Ascorbic Acid
This chemical diagram gives us a detailed look at how every atom in an ascorbic acid molecule is connected, which tells us its molecular formula. From there, figuring out the empirical formula is a pretty simple, step-by-step process.
Think of it this way: the molecular formula is the exact recipe, while the empirical formula is just the ratio of ingredients. Our job is to simplify that recipe down to its most basic ratio. This is more of a math puzzle than a chemistry one, but if you want a quick review, you can find a guide on how to solve math problems step-by-step at this link: https://feen.ai/blog/how-to-solve-math-problems-step-by-step
The molecular formula for ascorbic acid, C₆H₈O₆, was a huge discovery back in the early 1930s. Albert Szent-Györgyi first isolated what he called "hexuronic acid" from paprika, which we now know as Vitamin C. This breakthrough opened the door to understanding its vital role in our health.
Step-by-Step Calculation
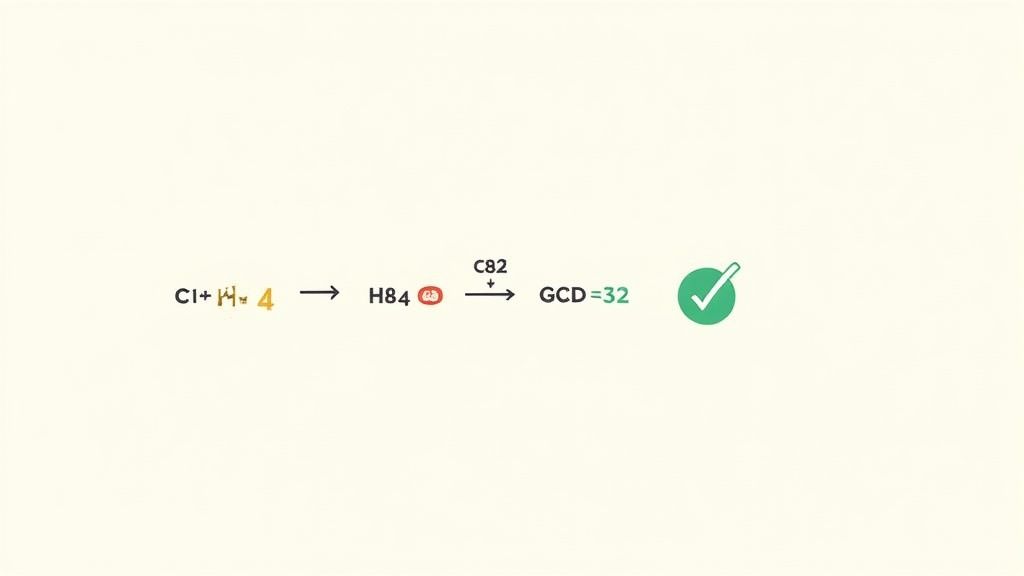
Alright, let's take the molecular formula for ascorbic acid—C₆H₈O₆—and find its simplest ratio.
List the Atom Counts: First, just look at the subscripts. We have 6 carbon atoms (C), 8 hydrogen atoms (H), and 6 oxygen atoms (O).
Find the Greatest Common Divisor (GCD): Now, what’s the largest number that divides evenly into 6, 8, and 6? A quick look tells us the GCD is 2.
Divide by the GCD: This is the key step. We just divide each subscript by our GCD, which is 2.
- Carbon (C): 6 ÷ 2 = 3
- Hydrogen (H): 8 ÷ 2 = 4
- Oxygen (O): 6 ÷ 2 = 3
Assemble the Empirical Formula: Use these new numbers as the subscripts. And there you have it: C₃H₄O₃.
The Final Answer: By simplifying the subscripts in the molecular formula C₆H₈O₆, we find that the empirical formula for ascorbic acid is C₃H₄O₃.
This same exact method works for any compound where you already know the molecular formula. It’s all about finding that common factor and simplifying. For those who need to gather this kind of data for their own work, knowing how to find reliable sources is crucial. It’s always a good idea to practice finding peer-reviewed scientific articles to ensure your information is accurate.
Why This Chemical Formula Matters for Our Health
Figuring out the formulas for ascorbic acid was much more than just a classroom exercise. It was a massive leap forward for nutritional science and public health, creating a direct line from the chemistry lab to our dinner plates and medicine cabinets.
Once scientists pinned down the molecular formula, C₆H₈O₆, they could finally identify and measure Vitamin C with precision. Before this, diseases like scurvy—a brutal condition caused by a lack of Vitamin C—were a serious global threat. By the 1930s, having the formula meant researchers could calculate the exact amounts people needed, which led to the Recommended Daily Allowances (RDAs) we still use today.
This breakthrough unlocked two game-changing developments:
- Food Fortification: Suddenly, governments and food companies could add specific amounts of Vitamin C to everyday foods. This simple act helped wipe out deficiency diseases on a huge scale.
- Mass Synthesis: Chemists now had the blueprint to create ascorbic acid in a lab, making it an affordable and easily accessible nutrient for the first time in history.
From Formula to Global Health
This ability to produce Vitamin C transformed it from a nutrient you could only get from certain foods into a global health staple. Today, it’s a powerhouse for supporting immune systems, keeping skin healthy, and fighting off damage as an antioxidant. It all started with a simple chemical formula.
The journey from identifying the empirical formula for ascorbic acid to creating the supplements on your shelf is a perfect example of how fundamental chemistry directly impacts our daily health and helps us live longer.
Of course, the story doesn't end with its structure. To learn more about how this molecule works in the body, you can explore the extensive benefits of Vitamin C supplements and see why it remains a cornerstone of modern wellness.
Common Questions About Ascorbic Acid's Formula
As you get more comfortable with chemistry, you'll notice a few questions about chemical formulas seem to pop up over and over again. Let's walk through some of the most common ones using ascorbic acid as our example to make sure the concepts are crystal clear.
Can a Molecule’s Empirical and Molecular Formulas Be the Same?
Absolutely. A great, simple example is water, H₂O.
The molecular formula tells us there are exactly two hydrogen atoms and one oxygen atom in a water molecule. Since you can't simplify a 2:1 ratio any further and still have whole numbers, the empirical formula for water is also H₂O.
How Do Scientists Find the Empirical Formula?
In a real lab, chemists often start without knowing the formula at all. They use a technique called elemental analysis to figure it out.
They take a pure sample of the substance, break it down into its basic elements, and then very carefully measure the mass of each element present. From there, they can convert those mass values into moles and calculate the simplest whole-number ratio—voilà, you have the empirical formula.
Why Does The Molecular Weight Matter?
Knowing the empirical formula is great, but for making something, you need the whole story. The full molecular formula for ascorbic acid, C₆H₈O₆, tells us its molecular weight is about 176.12 g/mol.
This exact number was historically crucial for chemists perfecting the synthesis of Vitamin C. It allowed them to get the "recipe" just right, ensuring production was efficient and the final product was pure.
Key Insight: The empirical formula gives you the simplest ratio. The molecular formula gives you the exact count of atoms and the true mass, which is what you need for any practical lab work or industrial manufacturing.
Ultimately, you need both formulas to get a complete picture of a compound. The logic of using one piece of information (like the empirical formula) to find another (the molecular formula) is a fundamental problem-solving skill in science, much like when you solve systems of linear equations by using knowns to find unknowns.
Struggling with complex chemistry problems or other tough homework assignments? Feen AI is your on-demand AI homework helper. Just upload a picture of your problem, and get clear, step-by-step solutions for chemistry, math, physics, and more. Get unstuck and study smarter at https://feen.ai.
Relevant articles
Discover an easy mass to moles conversion method with clear steps, practical examples, and pitfalls to avoid—boost your chemistry confidence today.
